Life From Chemicals — Fact of Fantasy?
 Did chemical reactions among randomly distributed molecules in the earth’s primordial ocean produce the first living cells?
Did chemical reactions among randomly distributed molecules in the earth’s primordial ocean produce the first living cells?
Little more than a century ago, science began to entertain notions of life arising from inert chemicals. Through the microscopes of that time, the cell appeared to be no more than a simple bag of chemicals. It therefore seemed reasonable to scientists such as Darwin to imagine that elementary living forms may have arisen from the random combination of organic chemicals in a primordial “soup.” But as man probed into the mysteries of the living cell, the idea that life came from chemicals began to appear less reasonable. Yet most scientists today cling to the dogma of chemical evolution.

If a scientist were reduced several million times in size, he could personally explore the complexity of life. Here the three-dimensional structure of a protein molecule in a living cell is represented by an arrangement of coils and curved arrows. The daunting intricacy of these molecules has come to symbolize the complexity of the living organisms. There are 2,000 kinds of proteins in the simplest bacteria and 800 times as many in a mammalian cell.
As time went on, microscopic exploration gradually revealed increasingly complex phenomena within the tiny cell, such as the precise regulation of cellular metabolism by the nucleic acids (DNA and RNA). which involves the sophisticated interaction of thousands of kinds of elaborately structured protein molecules. It was no longer quite so easy to imagine how all this could have occurred by random combination of chemicals.
Describing the remarkably intricate biochemistry of the cell, James D. Watson, co-discoverer of the DNA structure, wrote in his book Molecular Biology of the Gene. “We must immediately admit that the structure of the cell will never be understood in the same way as that of water or glucose molecules. Not only will the exact structure of most macromolecules within the cell remain unsolved, but their relative locations within cells can only be vaguely known. It is thus not surprising that many chemists, after brief periods of enthusiasm for studying life, silently return to the world of pure chemistry.”
Yet despite ever-increasing awareness of the structural and behavioral complexity of even the simplest living systems, many scientists continue to theorize that life has emerged from a primordial chemical soup without the direction of any higher organizing principles. They imagine that in the course of random chemical bonding, simple molecules combined into complex organic compounds, which eventually integrated themselves into self-reproducing organisms. This scenario is being presented as the undisputed truth about the origin of life in every science classroom around the world—in grade schools, high schools, and colleges and universities. Radio, television, and the popular science publications reinforce the message.
To some, talk about topics such as whether or not life emerged from matter may appear far removed from day-to-day affairs, and thus irrelevant to their own lives. Whether the discussions involve highly reasonable ideas based on solid evidence or vague, unsubstantiated hypotheses rooted in flimsy data and nurtured by scientific prejudice, they seem like subject matter for scholars in ivory towers. But because the answers to fundamental questions about the origin of life determine how we view ourselves and our place in the universe, they profoundly affect our sense of identity, our decisions, our feelings, our relationships, our behavior—in fact, they affect all aspects of our life, including the goals of our whole secular society.
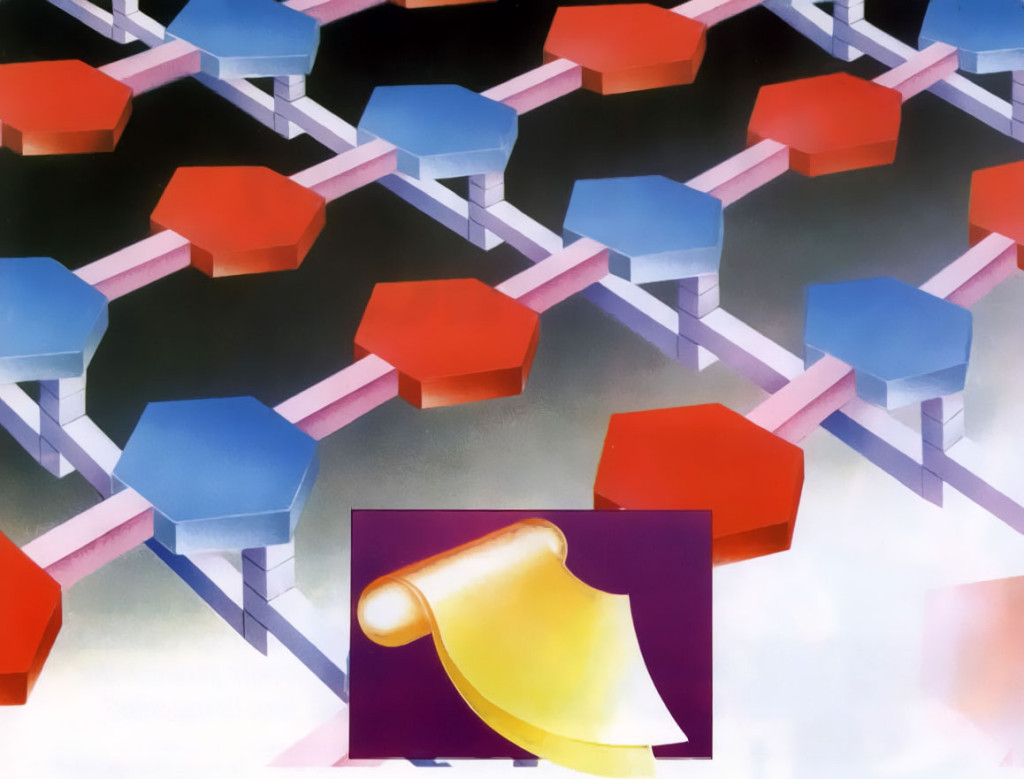
Fig. 1. A bacterial cell wall is made of layers of an intricate molecular fabric. The complexity of this structure challenges the idea it could have come about by natural selection or random chemical changes.
Before looking at the explanations offered by mechanistic theories on the origin of life and consciousness, we shall first consider three examples of what goes on inside the living cell, thereby helping us appreciate the incredible complexity of even the simplest organisms.
While contemplating these examples, it is crucial that we remember that according to the understanding of modern chemists, the molecules involved are merely submicroscopic units of matter. The remarkable ways in which they combine might lead one to attribute mystical potencies for self-organization to them. Scientists, however, are quick to reject this idea, insisting instead that molecules do nothing more than follow the laws of physics. But just how molecules acting according to these relatively simple mechanistic laws could combine together to produce inconceivably complicated cells has yet to be explained. And how such cells could evolve according to the same laws to produce complex higher organisms is an even knottier question. So despite the rigid adherence of the scientific community to its current mechanistic explanation of chemical evolution, it would seem appropriate for us to remain open to the possibility that other factors may be involved in chemical evolution—perhaps even some kind of self-intelligent organizing principle.
Our first example concerns the bacterial cell’s protective wall, which is manufactured from various molecules synthesized within the cell. To construct its wall, the cell initially forms molecular building blocks from simpler compounds by processes involving many sophisticated operations. Once these blocks are assembled, the cell arranges them into a precise weave of horizontal and vertical rows comprising the cell wall (see Fig. 1). This manufacturing process resembles a complex factory assembly operation, wherein specifically designed machines first build component parts from raw materials and then assemble those components into a functioning, finished product.
A second example of the cell’s internal complexity is its formation of a fatty acid, palmitic acid, from fourteen molecular sub-units. Fatty acids are the chief molecules for energy storage in cells. To manufacture palmitic acid, the cell creates an elaborate, circular “molecular machine” from protein molecules. At the “machine’s” center is an arm, also comprised of molecules, that swings through six “work stations”. Each time the arm rotates, two molecular subunits of the fatty acid are added by the action of enzymes at the work stations. (Enzymes arc highly complex protein molecules that aid chemical reactions within the cell.) After seven rotations, the required fourteen units arc present and the fatty acid is released.
For this rotary assembly machine to work, all six different enzymes must be present in the right order, and the molecular arm must be properly arranged. In general, a complex machine is operable only if all vital parts are present and functioning. For example, it would be hard to imagine an automobile engine being able to run without a fuel pump or camshaft. It’s hard to see, therefore, how the molecular machine described above could have come into being through any kind of step-by-step evolution.
Our third example, the action of the enzyme DNA gyrase in cellular reproduction, graphically illustrates the serious problems mechanistic theories face in attempting to explain the origins of complex behavior in cells. In a bacterium such as ecoli, the DNA molecule is a loop-shaped, intertwined double helix, which separates into two helixes during cellular reproduction. As the upper portion of the helix uncoils, it naturally causes the lower portion to wind upon itself, or supercoil. Since the DNA is already folded hundreds of times to fit in the cell, supercoiling invariably causes the strands to tangle. This tangling would prohibit reproduction: therefore the cell activates an enzyme, DNA gyrase, that unravels the knots in the DNA strands. The gyrase rearranges the DNA strands as follows. First it cuts one of the overlapping strands, then pulls the other strand through the opening, and finally joins the ends of the cut strand back together. By means of this highly sophisticated operation, the DNA gyrase sorts out the tangle of chromosomes (see Fig. 2).
The question for biochemists is this: How could the DNA gyrase molecule have originated? It must be much too complicated in structure to have come about in one stroke, by the random combinations of molecules in the primordial soup. Scientists might therefore suggest it underwent a process of gradual evolution, step by step. But here’s the catch—without DNA gyrase, there would have been no cellular reproduction, and without cellular reproduction, there is no evolutionary process to produce the gyrase. The origin of the gyrase enzyme thus remains one of the great mysteries of cellular evolution.
The above-mentioned three examples indicate the intricate structure and operation of the cell. No one has any experience of a machine that developed without a designer’s plan and specifications: therefore it’s reasonable to consider the possibility that such complex arrangements came about by a preconceived design. Unfortunately, such commonsense conclusions have no place in the currently dominant theories about the evolution of life. Rather, the proponents of chemical evolution struggle to manufacture alternative explanations that refer only to blind chance and the impersonal laws of physics.
The most common scenario portrayed by chemical-evolution theorists begins more than four billion years ago, when clouds of gases and dust are believed to have condensed on the earth’s ancient surface and gradually formed the primal atmosphere. Activated by ultraviolet light and electric bolts, this primitive atmosphere is supposed to have spontaneously given birth to organic chemical compounds, which then, for some 1.5 billion years, accumulated in ancient seas. These organic compounds interacted chemically and eventually formed primitive polypeptides (proteins), polynucleotides (DNA and RNA), polysaccharides (cell sugars), and lipids (fatty acids). A standard college text gives the final step: “From this rich broth of organic molecules and polymers, the primordial organic soup, the first living organisms are believed to have arisen. ”
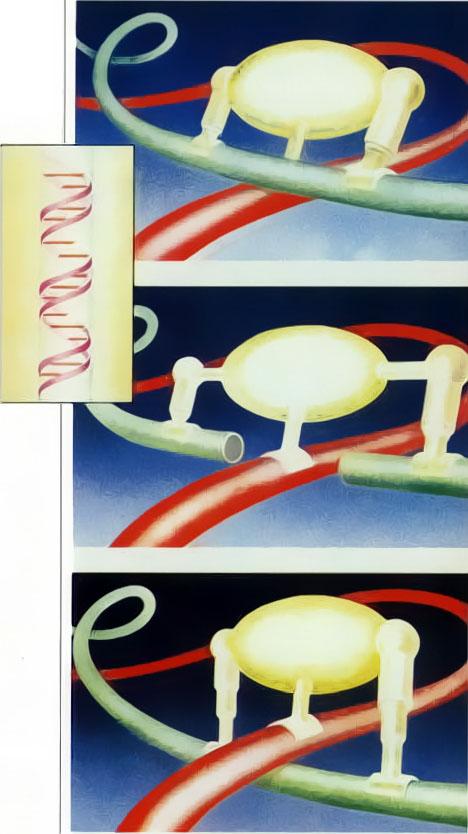
Fig. 2. The enzyme DNA gyrase can tie and untie knots in a cell’s DNA strands (colored tubes) by systematically breaking a strand, passing another strand through the break, and then resealing the break.
Unquestionably a provocative and somewhat poetic description—but how well does this grand speculation hold up to even moderate scrutiny? We have already discussed the amazing complexity of even simple living systems, so any claim that blind natural forces originally organized molecules into elaborately functioning systems must explain the exact principles and step-by-step processes involved. This has not been done.
Biochemists may call upon natural selection—the process whereby the varieties of an organism most suitably adapted to a particular environment tend to reproduce and survive—as an explanation. But natural selection cannot be proposed as a mechanism to account for the origin of the first living organism. It cannot act until such a self-replicating system actually exists, because without reproduction there are no new forms for nature to select. And given a simple self-replicating system, it is not enough for scientists to wave their hands and say the magic words “natural selection” in order to explain the appearance of more complex systems. They should be able to specify what exactly would be selected and why. Without being able to do this, they do not even have a theory to be tested and investigated, what to speak of a final demonstration of the truth of such a theory.
Unfortunately, present theories fail to approach this standard. Beginning with the work of Oparin in the 1930s, many scientists have made serious attempts to account for the origin of life from a primordial chemical soup, but none have been successful. Without exception, the models proposed are vague, tentative, incomplete, and sketchily worked out. We will discuss some but not all of these attempts. The central unresolved question is this: How could inert matter, acting according to simple physical laws alone, generate the remarkable molecular machinery found in even the simplest cell? As Albert L. Lehninger states in his widely used college biochemistry textbook, “At the center of the problem is the process of the self-organization of matter.” Yet up to now, scientists have failed to demonstrate how this could occur without the intervention of some higher directing force or intelligence.
Two especially well publicized experiments have frequently been misconstrued as being partially successful in producing life from chemicals. One is the work done with amino acids by Stanley Miller, a chemistry professor at the University of California at San Diego. The other is the “protocell experiments” of Sydney Fox. director of the Institute for Molecular and Cellular Evolution at the University of Miami in Coral Gables.
Miller sought to reconstruct conditions he believed existed at the “dawn of life” and thereby generate primitive organic forms from physical elements.
Into a flask he placed gases thought to comprise the ancient atmosphere, and by passing a spark through this mixture he produced a brown, tarry substance on the walls of the container. This tarry substance included amino acids, the constituents of protein molecules.
He heralded this as a significant breakthrough and managed to impress many people, both inside and outside the scientific community. Yet Miller’s experiments are actually of little, if any, significance. We would expect amino acids to form in Miller’s experiment, because this technique automatically produces practically every simple organic molecule found in nature (the vast majority of which are poisonous to present-day life forms). Asked to predict the outcome of Miller’s experiments, Harold Urey, a chemist at the University of California, put the whole affair into perspective when he replied, “Bielsteiny (Bielstein is the German catalog of all known organic chemicals.) Furthermore, amino acids are relatively simple molecules, serving merely as the building blocks of the far more complex protein molecules found in cells. It’s not surprising that a simple technique like Miller’s produces simple chemical results, but it has yet to be demonstrated that such a simple process can produce complex cellular components and mechanisms. It’s quite a step to go from unorganized building blocks to a house.
Chemist Sydney Fox also attempted to demonstrate how chemicals might progressively develop into a living cell. By heating dry amino acids to 280 degrees Fahrenheit and dropping them into water, he produced small drops of protein, which he optimistically labeled “protocells.” Fox’s protocells, however, were not overly impressive. Structurally, they were nothing more than hollow little globs of jelly, and they were incapable of metabolizing molecules from the environment. They showed no signs of evolving into even slightly more complex forms, what to speak of cells. On top of all this, Fox has no reasonable suggestion as to how they could have emerged from a pre-biotic chemical soup. (Getting dry amino acids heated to 280 degrees in nature requires quite a bit of imagination.) There are many other experiments like this that produce similar results and leave the same questions unanswered.
German scientist Manfred Eigen has proposed an explanation of how inert chemicals might make the transition to self-reproducing cells. According to Eigen. several kinds of RNA molecules would replicate individually in the primordial soup. For instance, type A would replicate RNA of type A. and type B would replicate more RNA of type B. These cycles would go on independently of each other. But then somehow, according to Eigen, the A-type RNA cycle would begin to produce an enzyme E-B that would catalyze the replication of the B-type RNA. And also the B-type RNA would begin to produce an enzyme E-A that would catalyze the replication of the A-type RNA. With the production of these enzymes, the A-B-A-B-A-B cycle would continue. This is called a hypercycle. and Eigen proposes that the hypercycles could gradually become more and more complex until they approached the level of living cells.
There are, however, major problems with hypercycles. First, the model requires a mechanism for producing complicated proteins (in the form of enzymes) from information coded in the RNA. Eigen has not been able to suggest a workable mechanism of this kind. Second, given a function hypercycle, there is no certainty it would evolve. The prominent evolutionary biologist John Maynard Smith critized Eigen’s model, pointing out that unless the hypercycle were enclosed within a compartment resembling a cell wall its different parts would compete with each other this would make it impossible for the hypercycle itself to evolve by mutation and natural selection. And if the need for the compartment is admitted, there remains the difficult problem of accounting for the apparatus by which it could replicate itself during reproduction. Smith says, “Clearly these papers [of Eigen and his coworkers] raise more problems than they solve.”
Finally hypercycles are much different than cells, which have a unified genetic system and complected molecular mechanisms. To go from a hypercycle to a cell would take thousands of intermediate steps. It would be like going from a wind-up clock to an internal combustion engine by small changes. Each change would have to result in an improved and functioning mechanism—a possibility that at present defies imagination. In his appeal to natural selection. Eigen does not define the exact steps that would lead from his hypercycles to living cells, and therefore his explanation amounts to no more than an unscientific wave of a magic wand.
Thus far we have seen how cells function in a remarkably organized manner and how the leading theories that attempt to describe the development of living cells from inert chemicals lack any explanatory value. At this point, we may ask why scientists persist in their attempt to find strictly mechanistic explanations. One answer is that they feel committed to their present reductionists strategy, which is to explain everything—from galaxies to bacteria—in terms of matter acting according to basic, simple laws of physics. Rejecting the possibility of any other approach to science, they fear that to deviate even slightly from their strategy would lead to the end of science as they know it.
Being unable to provide any suitable mechanism for the formation of the cell by simple physical laws, many scientists have turned to “chance” as the ultimate causative factor. There is. however, a fundamental problem with this approach. Strictly speaking, the term chance refers only to the presence of certain patterns in the statistics describing the repetitions of an event: it cannot be the “cause” of anything (see “Chance and the Origin of the Universe” on page 9). As for the mathematical probability of life arising from matter, there are some easily calculated estimates of the chance of such an event occurring over the course of 4.5 billion years, the age of the earth given by modern science.
Let’s begin by looking at the basic ingredient of all living organisms—proteins which carry out many of the vital functions of the cell. Proteins are formed in a highly complex process that can be compared to a factory assembly line, where raw materials are organized with the help of specialized machines. The elaborate protein macro-molecules contain an average of 300 amino acid molecules linked in a chain, and within even the simplest E. co/i bacteria there are approximately 2.000 different types of proteins. (In mammals there are 800 times as many.) The formation of these different protein molecules is controlled by the cell’s genetic material. According to a mechanistic model, prior to the development of a self-reproducing system capable of performing the basic functions of a cell and its genetic coding, any combining of amino acids into proteins would have necessarily been due to random interaction.
To determine the probability of random interaction resulting in the proteins required for even the simplest cell, the noted British astronomer Sir Fred Hoyle and mathematician Chandra Wickramasinghe. of University College. Cardiff. Wales, calculated as follows. As already mentioned, there are 2,000 different proteins necessary for the single-celled E. coli bacteria, and these proteins average 300 amino-acid units in length. The function of a particular protein depends upon the sequential order ofits 300 or so amino-acid units, just as the meaning of a paragraph depends on the order of its words. Since there are 20 amino-acid types to choose from, the odds of forming any particular protein sequence is 20300 to 1.
Scientists have pointed out that there is some latitude for variation in the exact sequence of the 300 amino acid units without disrupting the protein’s performance. Therefore Hoyle and Wickramasinghe generously adjusted the 20300 to 1 probability to 1020 to 1—a tremendous reduction in the odds. Then, since the simplest cell requires 2,000 different proteins to operate, they combined these two figures (1020 and 2,000) and arrived at a mathematical probability of lO40000 to 1 that random interaction could provide the necessary molecules for constructing even the simplest self-reproducing system. These odds are so incredibly great that no one could reasonably expect such an event to occur in the relatively brief few billion years that scientists allow for the phenomenon (see “Could Life Arise by Chance?” below). So much for pure chance.
Many scientists dislike this concept of chance, but they have concluded that as far as their present mechanistic understanding is concerned, it looks as though life must have originated by a “chance event” of extremely small probability. One of these is Nobel laureate Francis Crick, codiscoverer of the DNA structure, who stated, “An honest man, armed with all the knowledge available to us now, could only state that in some sense, the origin of life appears at the moment to be almost a miracle, so many are the conditions which would have had to have been satisfied to get it going.”These scientists have of course hoped to explain the origin of life on the basis of natural laws. But as we have seen, they have been unable to do so. Thus stymied, some of these scientists have turned to extremely radical hypotheses (but of course not so radical as the concept of a designer).
For example, Crick himself has proposed that the genetic code may have been carried to earth by intelligent life from another planetary system. This concept could account for life on earth, but we are then left lo explain how life developed elsewhere.
It is clear that there is no viable theory of the chemical origin of life.
So although vast numbers of people believe that science has substantial evidence “proving” the idea that the first living entities were produced from the random interaction of chemicals in the earth’s distant past, it is clear that there exists no viable theory of the chemical origin of life.
Furthermore, the mathematical theory of probability does not allow us to use the convenient explanation “It happened by chance.”
Therefore, because there is nothing even approaching a mechanistic explanation for the high information content of living systems, we propose that living organisms can’t be explained in mechanistic terms. In “The Mystery of Consciousness,” we discussed an irreducible, nonmechanistic aspect of reality, namely consciousness. Now we have another irreducible aspect of reality that cannot be accounted for by mechanistic science—namely, the complex forms of living organisms. We propose that a superconscious intelligence is responsible for both of these phenomena. It is the original source of the conscious entities within physical organisms and provides the information for the arrangement of matter into the biological structures that serve as vehicles for those conscious entities. The nature of this higher intelligence will be more elaborately discussed in the final article in this magazine, “Higher Dimensional Science.”















